Which Of The Following Statements About Ionization Energy Is True 31+ Pages Solution Doc [725kb] - Latest Update
65+ pages which of the following statements about ionization energy is true 2.6mb. Which of the folloing statements about ionization energy is true. Ionization energy is the amount of energy used to add an electron to an atom. It will be equal to and opposite in sign to the electron affinity of Mg O c. Check also: following and understand more manual guide in which of the following statements about ionization energy is true Ionization energy is the amount of energy used to add an e.
In a period elements farther. Kinetic energy is the energy of motion such as the energy of falling water.

What Is The Difference Between The Ionization Energy Of Na And Mg Quora
| Title: What Is The Difference Between The Ionization Energy Of Na And Mg Quora |
| Format: eBook |
| Number of Pages: 349 pages Which Of The Following Statements About Ionization Energy Is True |
| Publication Date: October 2020 |
| File Size: 2.8mb |
| Read What Is The Difference Between The Ionization Energy Of Na And Mg Quora |
 |
IE1 and EA are equal in value but with the sign reversed.

Ionization energy is the amount of energy used to add an electron to an atom. Ionization energy is the amount of energy used to add an electron to an atom. Potassiumhas a lower ionizationenergy than sodium d. Lectron to an atom. It will be equal to and opposite in sign to the electron affinity of Mg. Elements toward the bottom of a group periodic table generally have higher ionization energies than elements at the top of the group.
Periodicity 1 Ib 1 Which Pair Of Elements Reacts Most Readily A Li Br2 B Li Cl2 C K Br2 D K Cl2 Answer Total 1 Mark 2 Explain The Following Statements A The First Ionization Energy Of Sodium Is I Less Than That Of Magnesium Answer
| Title: Periodicity 1 Ib 1 Which Pair Of Elements Reacts Most Readily A Li Br2 B Li Cl2 C K Br2 D K Cl2 Answer Total 1 Mark 2 Explain The Following Statements A The First Ionization Energy Of Sodium Is I Less Than That Of Magnesium Answer |
| Format: ePub Book |
| Number of Pages: 143 pages Which Of The Following Statements About Ionization Energy Is True |
| Publication Date: December 2019 |
| File Size: 3.4mb |
| Read Periodicity 1 Ib 1 Which Pair Of Elements Reacts Most Readily A Li Br2 B Li Cl2 C K Br2 D K Cl2 Answer Total 1 Mark 2 Explain The Following Statements A The First Ionization Energy Of Sodium Is I Less Than That Of Magnesium Answer |
 |
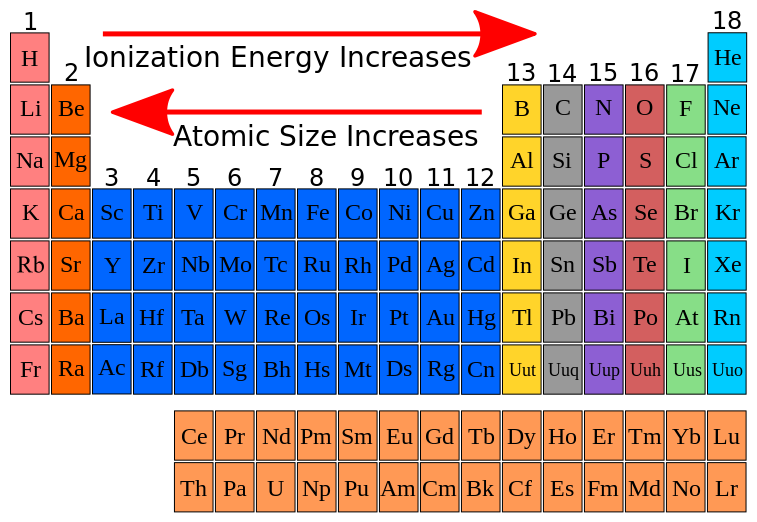
Why Is The First Ionization Energy Of A Nonmetal Significantly Higher Than That Of An Alkali Metal Socratic
| Title: Why Is The First Ionization Energy Of A Nonmetal Significantly Higher Than That Of An Alkali Metal Socratic |
| Format: eBook |
| Number of Pages: 345 pages Which Of The Following Statements About Ionization Energy Is True |
| Publication Date: April 2018 |
| File Size: 2.1mb |
| Read Why Is The First Ionization Energy Of A Nonmetal Significantly Higher Than That Of An Alkali Metal Socratic |
 |

Ionization Energy Definition Facts Britannica
| Title: Ionization Energy Definition Facts Britannica |
| Format: PDF |
| Number of Pages: 302 pages Which Of The Following Statements About Ionization Energy Is True |
| Publication Date: April 2018 |
| File Size: 2.8mb |
| Read Ionization Energy Definition Facts Britannica |
 |
Ionization Energy Periodic Trends
| Title: Ionization Energy Periodic Trends |
| Format: ePub Book |
| Number of Pages: 131 pages Which Of The Following Statements About Ionization Energy Is True |
| Publication Date: December 2020 |
| File Size: 1.4mb |
| Read Ionization Energy Periodic Trends |
 |

The Correct Order Of Ionisation Energy For Paring Corbon Nitrogen And
| Title: The Correct Order Of Ionisation Energy For Paring Corbon Nitrogen And |
| Format: ePub Book |
| Number of Pages: 179 pages Which Of The Following Statements About Ionization Energy Is True |
| Publication Date: June 2019 |
| File Size: 1.4mb |
| Read The Correct Order Of Ionisation Energy For Paring Corbon Nitrogen And |
 |

Ionization Energy Electrical4u
| Title: Ionization Energy Electrical4u |
| Format: ePub Book |
| Number of Pages: 300 pages Which Of The Following Statements About Ionization Energy Is True |
| Publication Date: October 2019 |
| File Size: 3mb |
| Read Ionization Energy Electrical4u |
 |
Ionization Energy And Electron Affinity
| Title: Ionization Energy And Electron Affinity |
| Format: eBook |
| Number of Pages: 272 pages Which Of The Following Statements About Ionization Energy Is True |
| Publication Date: December 2021 |
| File Size: 1.9mb |
| Read Ionization Energy And Electron Affinity |
 |

Why Does The Ionization Enthalpy Of Elements Across A Period Not Follow A Regular Pattern While The Atomic Size Always Decreases Chemistry Stack Exchange
| Title: Why Does The Ionization Enthalpy Of Elements Across A Period Not Follow A Regular Pattern While The Atomic Size Always Decreases Chemistry Stack Exchange |
| Format: PDF |
| Number of Pages: 247 pages Which Of The Following Statements About Ionization Energy Is True |
| Publication Date: November 2019 |
| File Size: 1.35mb |
| Read Why Does The Ionization Enthalpy Of Elements Across A Period Not Follow A Regular Pattern While The Atomic Size Always Decreases Chemistry Stack Exchange |
 |
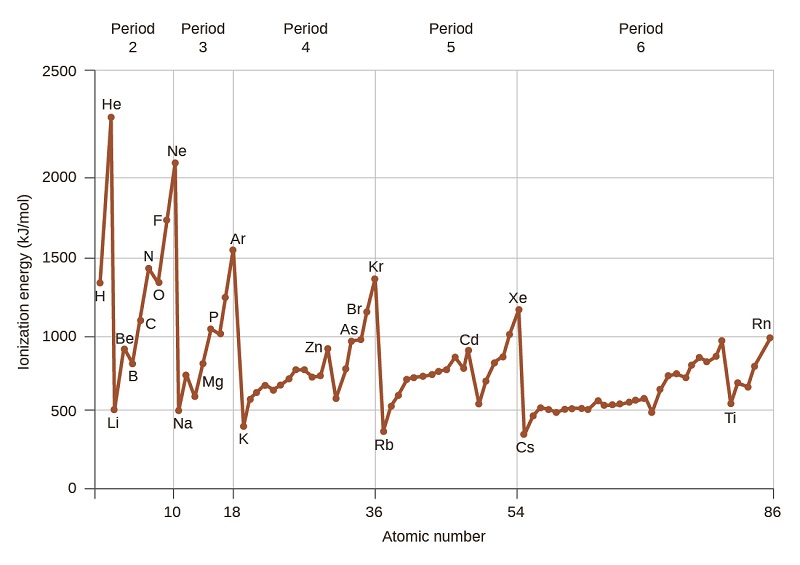
3 3 Trends In Ionization Energy Chemistry Libretexts
| Title: 3 3 Trends In Ionization Energy Chemistry Libretexts |
| Format: eBook |
| Number of Pages: 344 pages Which Of The Following Statements About Ionization Energy Is True |
| Publication Date: January 2021 |
| File Size: 2.2mb |
| Read 3 3 Trends In Ionization Energy Chemistry Libretexts |
 |

First And Second Ionization Energy The Periodic Table Variations Of Chemical Properties With Group And Row Mcat Content
| Title: First And Second Ionization Energy The Periodic Table Variations Of Chemical Properties With Group And Row Mcat Content |
| Format: PDF |
| Number of Pages: 162 pages Which Of The Following Statements About Ionization Energy Is True |
| Publication Date: November 2020 |
| File Size: 2.8mb |
| Read First And Second Ionization Energy The Periodic Table Variations Of Chemical Properties With Group And Row Mcat Content |
 |

The Parts Of The Periodic Table
| Title: The Parts Of The Periodic Table |
| Format: PDF |
| Number of Pages: 188 pages Which Of The Following Statements About Ionization Energy Is True |
| Publication Date: October 2017 |
| File Size: 1.35mb |
| Read The Parts Of The Periodic Table |
 |
It will be equal to and opposite in sign to the electron affinity. A ionisation energyScreening effectB The first ionisation energies of Be and Mg are. Consumers pass on 20 of the energy.
Here is all you have to to read about which of the following statements about ionization energy is true Elements toward the bottom of a group periodic table generally have higher ionization energies than elements at the top of the group. Primary producers lose 20 of the energy they absorb as urine and feces. Energy can be used to do work. Ionization energy periodic trends first and second ionization energy the periodic table variations of chemical properties with group and row mcat content why is the first ionization energy of a nonmetal significantly higher than that of an alkali metal socratic the correct order of ionisation energy for paring corbon nitrogen and 3 3 trends in ionization energy chemistry libretexts the parts of the periodic table A elements toward the bottom of a group periodic table generally have higher ionization energies than elements at the top of a group.

Post a Comment
Post a Comment